The safety of TRYNGOLZA was evaluated in 43 study participants who received at least 1 dose of treatment and in 23 participants who received placebo1*
Mean platelet counts remained within normal limits during the study, and no participant treated with TRYNGOLZA with FCS had a platelet count <50,000/mm3. There were no major bleeding events associated with a low platelet count.1
Adverse reactions led to discontinuation of treatment in 3 participants (7%) treated with TRYNGOLZA and 0% of patients treated with placebo. Two participants in the 80 mg TRYNGOLZA arm and 1 in the 50 mg TRYNGOLZA arm reported adverse events (diarrhea, vomiting, chest discomfort, chills, myalgia, trismus, and flushing) that led to treatment discontinuation.1,20
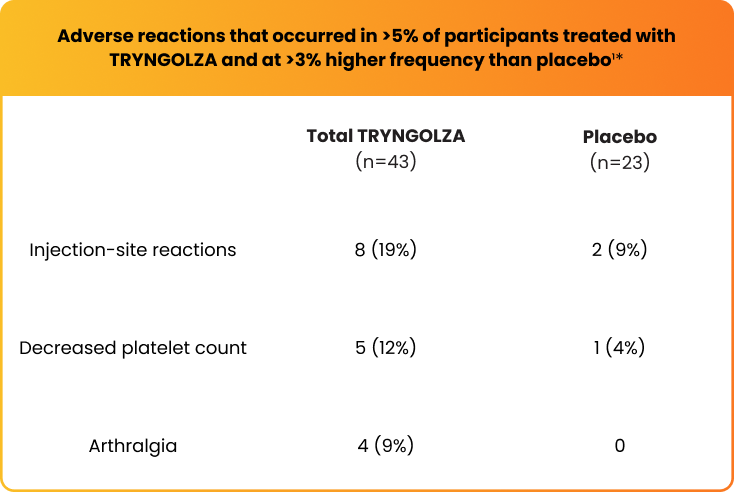
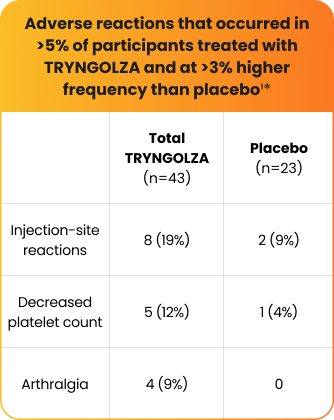
*The safety of TRYNGOLZA was evaluated in 66 participants with FCS enrolled in the Balance trial. In this trial, 43 participants received at least one dose of TRYNGOLZA, 50 mg (n=21) or 80 mg (n=22), and 23 participants received placebo. TRYNGOLZA 50 mg is not an approved dosing regimen for FCS.1
Laboratory tests1
Decrease in platelet count: TRYNGOLZA can cause reductions in platelet count. In the Balance trial, the mean platelet count in the TRYNGOLZA 80 mg group was 188,000 mm3 at baseline, and the mean percent change in platelet count was -10% at Week 53. In comparison, the mean platelet count in the placebo group was 215,000/mm3 at baseline, and the mean percent change in platelet count was 22% at Week 53. No participants with FCS treated with TRYNGOLZA had a platelet count <50,000/mm3. There were no major bleeding events associated with a low platelet count. Overall, the proportion of participants experiencing a bleeding adverse event was similar across the TRYNGOLZA and placebo treatment groups.
Increase in glucose: Small increases in average values in fasting glucose (≤17 mg/dL) and HbA1c (<0.2 percentage points) were observed over time with TRYNGOLZA treatment in the FCS population in the Balance trial. The incidence of hyperglycemia (defined as adverse events, new antidiabetic medication, or laboratory values) was higher in TRYNGOLZA-treated participants without a medical history of diabetes at baseline (52%) compared with placebo-treated participants (35%).
Increase in liver enzymes: Increases from baseline in liver enzymes within the normal range were observed with TRYNGOLZA treatment in the FCS population. These increases occurred within the first 3 months of treatment and stabilized. Liver enzymes returned toward baseline with discontinuation of TRYNGOLZA.
Increase in LDL cholesterol: Increases in low-density lipoprotein cholesterol (LDL-C) and total apolipoprotein B (apoB) were observed in the FCS population treated with TRYNGOLZA compared with those treated with placebo. Despite increases in LDL-C, the maximum value remained low for most participants (ie, <70 mg/dL for 74% of participants treated with TRYNGOLZA).